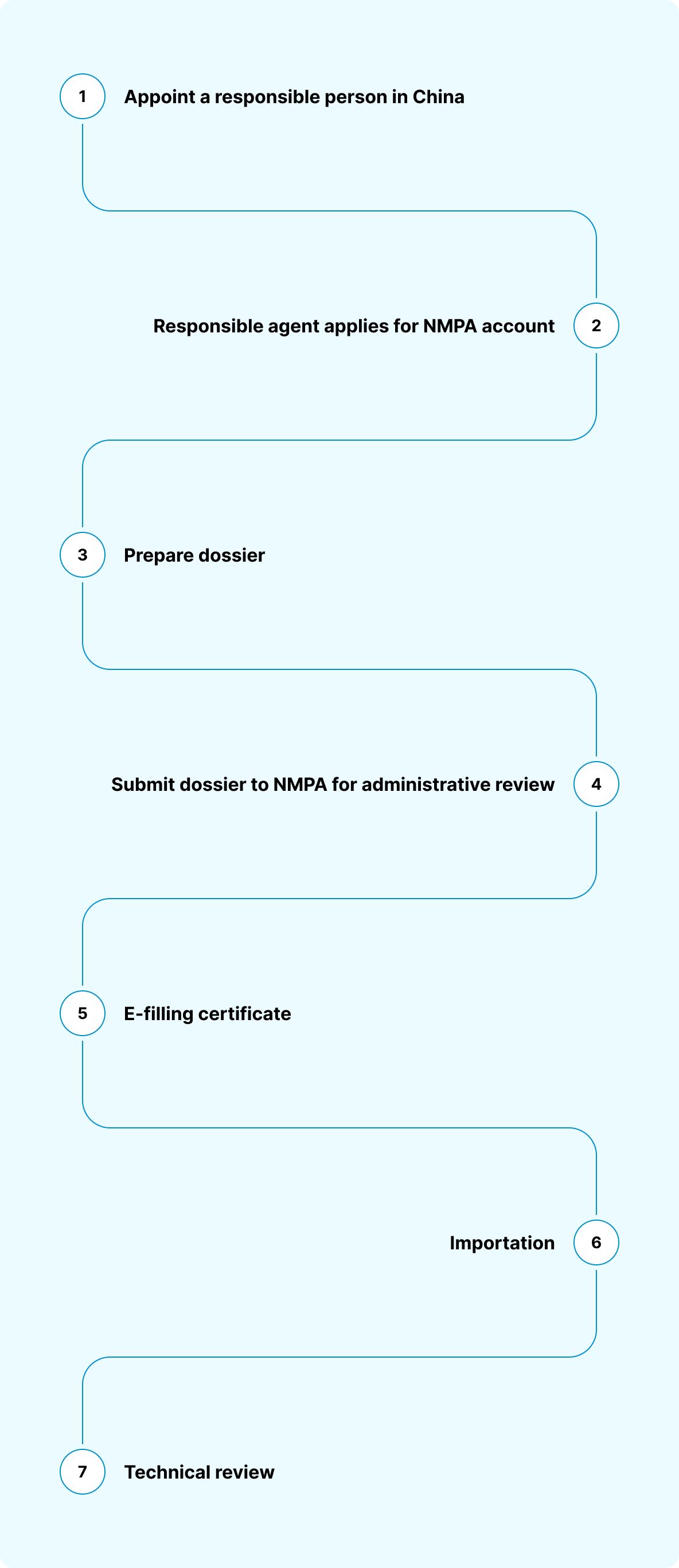
Applicable legislation
Cosmetic regulations in China are supervised by 2 main competent authorities: State Administration for Market Regulation (SAMR) and the independent Drug Administration Bureau called National Medical Products Administration (NMPA), which is under the governance of SAMR. Furthermore, NMPA consists of 9 subordinate departments, including Cosmetic Safety Supervision department. At provincial level and under NMPA, there are Medical Products Administrations (MPAs), which are in charge of filing of domestic non-special use cosmetics and issuance of cosmetics manufacturers' production license.
The current regulatory system in China is founded on the Regulations concerning the Hygiene Supervision over Cosmetics from 1989. There are multiple laws that have to be followed and taken into account in China, but the most important is the Technical Safety Standard for Cosmetics 2015 which replaced the Hygiene Standard for Cosmetics 2007.